Active Clinical Trials and Network Grants
SIREN
Network for Emergency Care Clinical Trials: Strategies to Innovate EmeRgENcy Care Clinical Trials Network

NIH NHLBI NINDS U24NS100673-06 (MPI: Merck- contact, Wright, Papa, Selker, Mosier, Jones)
2/1/2023 – 1/31/28
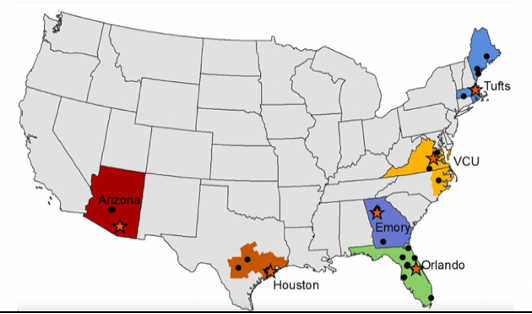
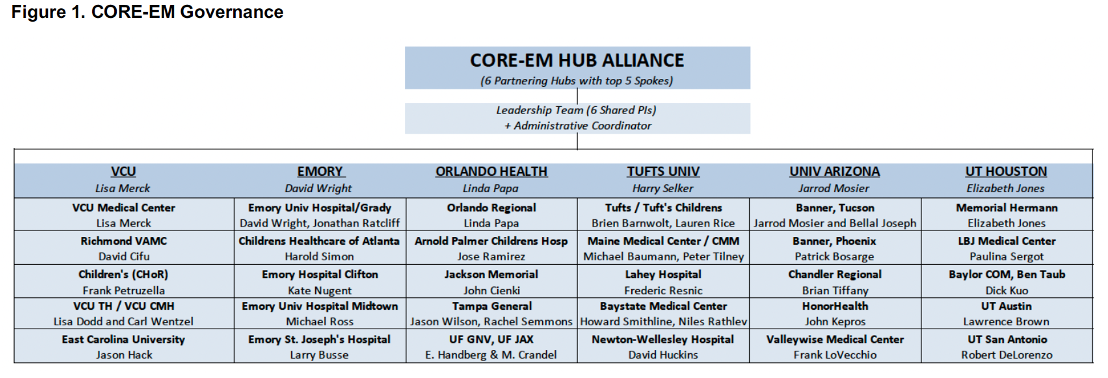
SIREN | CORE-EM Hub Alliance: VCU is the contact Hub for the CORE-EM Hub Alliance. This is one of 12 Hubs in the NIH funded SIREN clinical trial network. CORE-EM is composed of Emory, U Arizona, U Texas, Orlando Health, Tufts, and VCU. CORE-EM provides access to clinical trials across a combined population composed of over 72 million ethnically and racially diverse people across the US. Through SIREN, VCU will participate in clinical trials to study novel therapies for time-critical acute conditions such as traumatic brain injury, stroke, cardiac arrest, and sepsis. SIREN Fellows 2023-2024: Dr. Michael Joyce and Dr. Megan Donohue.

ICECAP
Influence of cooling duration on efficacy in cardiac arrest patients (ICECAP)

NIH / NINDS / NHLBI 1UG3HL145269-01A1 (MPI: Meurer, Romergryko, Silbergleit)
9/19/2019 – 6/31/2024
Registered with ClinicalTrials.gov: NCT04217551
NIH Project Number: UH3HL145269
Status: Enrolling
Neurological death and disability are common outcomes in survivors of cardiac arrest. Therapeutic cooling of comatose patients resuscitated from shockable rhythms markedly increases the rate of good neurological outcome, but poor outcomes still occur in as many as 50%, and the benefit of cooling in those resuscitated from asystole and pulseless electrical activity has not been shown in a randomized study.
The Influence of Cooling duration on Efficacy in Cardiac Arrest Patients (ICECAP) study will enroll comatose adult survivors of out of hospital cardiac arrest that have already been rapidly cooled using a definitive temperature control method. Those with and without initial shockable rhythms will be studied as distinct populations (maximum of 1800 subjects over four years). ICECAP will determine if identifying an optimal duration of cooling can improve outcomes, and if development of a duration response curve can substantiate efficacy in a wider patient population.
VCU Collaborative Investigator Team: </u > Lisa H. Merck MD MPH (EM), Joseph Ornato (EM), Mimi Peperdy (Cardiology/CICU); Michael Joyce MD (EM); Megan Donohue MD MPH (EM/IM); Noah Hillerbrand</em > MD (EM Resident); Shraddha Mainali MD (Neurology/NCC); Danielle Gong MD (EM/Informatics); Vishal Yajnik MD (Anesthesia/NCC); Michelle Gossip RN (CICU)</em >
Upcoming:
POST-ICECAP – extended outcomes after ROSC
BOOST3
Brain Oxygen Optimization in Severe TBI Phase-3

Registered with ClinicalTrials.gov: NCT03754114
NIH Project Number: 1U01NS099046-01A1
International, 60 center, adult and pediatric randomized clinical trial to assess the effect of Pbt02 in goal directed therapy for severe TBI.
07/01/2018 – 06/30/2023
Status: Enrolling
Traumatic brain injury (TBI) is a major cause of death and disability in developed societies. Every year, approximately 3.5 million Americans sustain a TBI, of which 50,000 die, and another 300,000 are hospitalized and survive the injury. BOOST3 is a randomized clinical trial to determine the comparative effectiveness of two strategies for monitoring and treating patients with traumatic brain injury (TBI) in the intensive care unit (ICU). The study will determine the safety and efficacy of a strategy guided by treatment goals based on both intracranial pressure (ICP) and brain tissue oxygen (PbtO2) as compared to a strategy guided by treatment goals based on ICP monitoring alone. Both of these alternative strategies are used in standard care. It is unknown if one is more effective than the other. In both strategies the monitoring and goals help doctors adjust treatments including the kinds and doses of medications and the amount of intravenous fluids given, ventilator (breathing machine) settings, need for blood transfusions, and other medical care. The results of this study will help doctors discover if one of these methods is more safe and effective.
VCU Collaborative Investigator Team: Lisa H. Merck MD MPH (EM); John Greer MD PhD (Neurosurgery); Michael Joyce MD (EM); Megan Donohue MD MPH (EM/IM); Noah Hillebrand MD (EM Resident); Nora Poulus MD (Neurosurgery Resident); Shraddha Mainali MD (Neurology/NCC); Chris Hogan (EM/NCC); Dennis Rivet MD (Neurosurgery); Sayuri Jinadasa MD (Trauma Surgery); Sydney Radding, MD (Trauma Surgery); Gretchen Brophy PhD (Pharmacy/NCC)
Upcoming:
BioBOOST - Biomarkers in the Brain Oxygen Optimization in Severe TBI Trial (Bio-BOOST)
DOD, USAMRAA W81XWH1910829 (MPI: Diaz, Korley)
Traumatic brain injury (TBI) remains a major cause of death and disability, with an estimated cost of $45 billion/year in the United States alone. Patients with severe TBI, defined as those who are unconscious or only minimally responsive to external stimulation, have an especially poor prognosis. Approximately 35% of patients with severe TBI die within 6 months of injury and among those who survive, approximately 80% have life-long disabilities, representing a large unmet medical need. One of the critical barriers to progress in discovering new treatments for severe TBI is the lack of blood tests that can guide the personalization of TBI treatments so that the right treatment is administered to the right type of TBI patient. Additionally, there are no blood tests for monitoring ongoing brain injury and individual patient response to treatment. The recently funded BOOST-3 (Brain Oxygen Optimization in Severe TBI Phase 3) trial offers a unique opportunity to study and validate new blood tests for TBI. BOOST-3 will enroll 1094 participants with severe TBI from 2019 – 2023, across 47 clinical sites in the US, and represents a $32.5 M federal investment. BOOST-3 will examine whether severe TBI treatment based on values obtained brain tissue oxygen and intracranial pressure values obtained from probes inserted into the brain will improve neurologic outcome at 6 months after injury compared to treatment based on intracranial pressure monitoring only. Capitalizing on the infrastructure and the rich study population for BOOST-3, we propose conducting an ancillary biomarker study, Bio-BOOST. The main goal of Bio-BOOST is to determine how the lack of oxygen to brain tissues affects the levels of blood tests for brain injury.
Publications related to BOOST3:
https://pubmed.ncbi.nlm.nih.gov/35273066/
Brain Oxygen Optimization in Severe Traumatic Brain Injury (BOOST-3): A multicenter, randomized, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. Bernard F MD; Barsan W MD; Diaz-Arrastia R MD PhD; Merck LH MD MPH; Yeatts S PhD; Shutter L MD. British Medical Journal Open, BMJ Open 2022;12:e060188. doi:10.1136/bmjopen-2021-060188
under construction