Mission
Our research mission is to provide outstanding clinical care through excellence in research and discovery, while mentoring future generations of clinician scientists.
Clinical research in the Department of Emergency Medicine extends from the field and emergency medical services through the front-door of the hospital, the intensive care units, operating rooms, and into outpatient rehabilitation. We provide clinical care and access to clinical research 24/7/365.
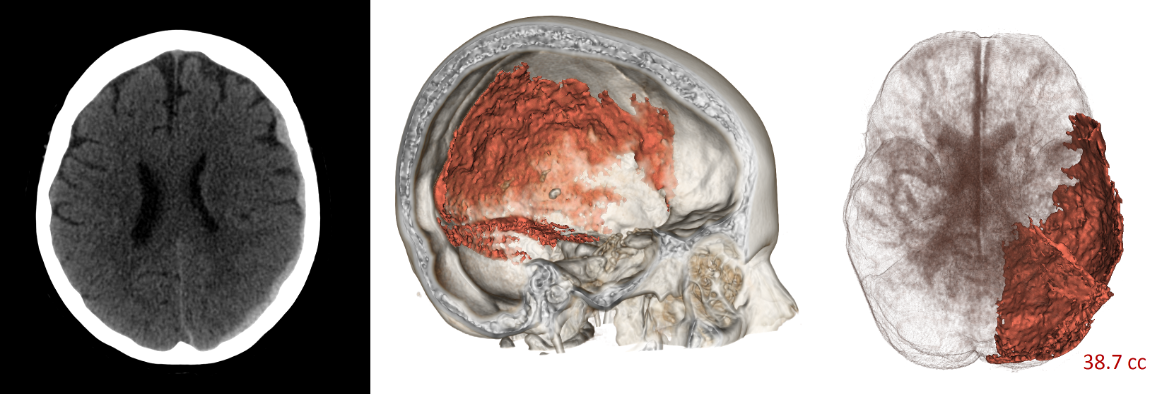
Faculty in the department hold a diverse range of research expertise in pediatric and adult emergency medicine, toxicology, EMS, clinical trials, education, ethics, critical care, engineering, public service, and biomarker development. Our faculty serve as leaders in clinical and translational research in traumatic brain injury, cardiac arrest, and infectious disease, working to design and execute research that will improve clinical care through evidence-based outcomes assessment. Our mission supports the education of all learners ~ undergraduate, graduate, post-doctoral, and scientific professionals are welcome to contact the team about opportunities for collaboration.